Zinc is a chemical element with the symbol Zn and Atomic number 30. Zinc is a slightly brittle metal at room temperature, and when it is deoxidized, it obtains a silver gray color. It is the first element in the 12th family (IIB) of the periodic table. Hydroxide is any compound containing one or more functional groups, containing an atom that combines with oxygen and hydrogen as negatively charged ions. The positively charged part of a compound can usually be an organic group (such as guanidine or tetramethylammonium), but it is usually a metal ion (such as sodium, magnesium, or aluminum).
What is Zinc hydroxide?
Zinc hydroxide (Zn (OH) 2) is a Inorganic compound. It also exists naturally in the form of three rare minerals: ws: 1rhombic pyroxene (orthorhombic), ashoverite, and steatite (all tetragonal).
Zinc hydroxide (and zinc oxide) is amphoteric, just like the hydroxides of other metals such as lead, aluminum, beryllium, tin and chromium. Therefore, it is easily soluble in alkaline solutions, such as sodium hydroxide, and in dilute solutions of strong acids, such as hydrochloric acid.
Zinc hydroxide exists on the earth as a rare natural mineral. It is also an amphoteric white solid that is soluble in solutions of strong acids or bases. It exists in the form of three Rare-earth mineral: silver dolomite, vanadium molybdenum ore, etc. Its chemical formula is Zn (OH) 2.
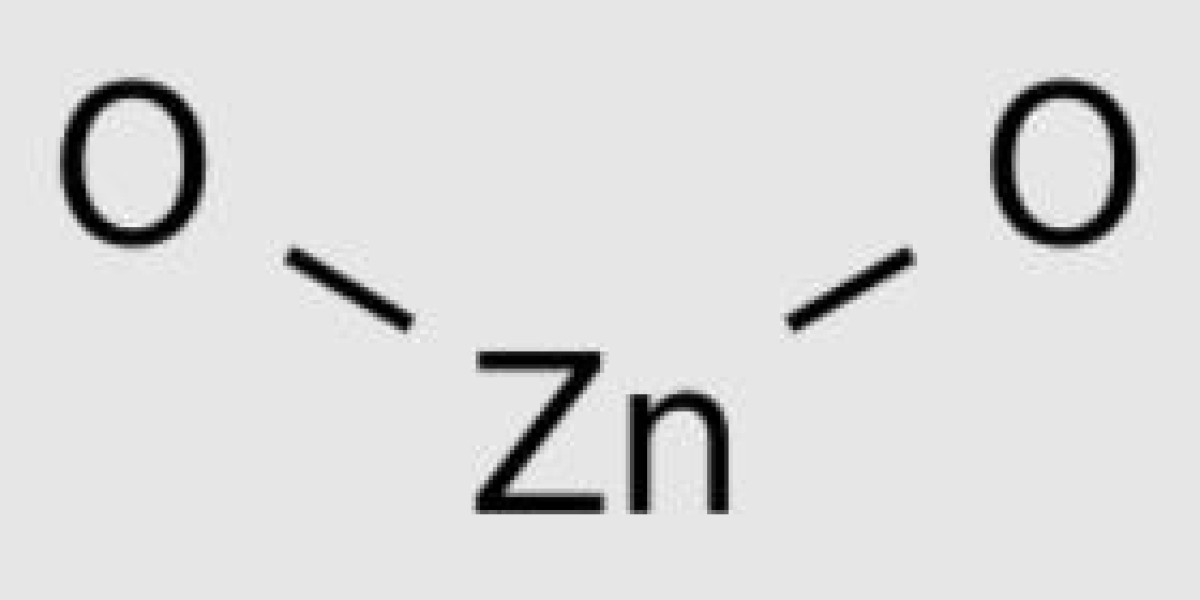
Structure of Zinc hydroxide
Chemical formula of Zinc hydroxide or Zinc hydroxide: Zn (OH) 2. It exists in a square or orthogonal form.
Preparation of Zinc hydroxide
Prepare by adding a small amount of sodium hydroxide solution into any zinc salt solution.
Zn2++2 OH - Zn (OH) 2
Zn2+forms high concentration water ions and tetrahydrate ions at low water concentration. This reaction can be written as water ions in the form of water ions, and the reaction with Hydroxide ion can be written as water ions in the form of water ions. The donation of protons is as follows:
Zn2+(here) 4 (aq)+oh - (aq) Zn2+(here) 3 oh - (aq)+water (left)
When sodium hydroxide is added, the sodium hydroxide will remain dissolved when sodium hydroxide is added. Colorless ionic zinc water:
Zn (OH) 2+2 OH - Zn (OH) 42-
Ions are naturally surrounded by water ligands, so zinc dissolves in water. Excess sodium hydroxide is added to the solution, and sodium hydroxide ion reduces the composite to two rates and dissolves. When ammonia is added to the excess solution, the balance of Hydroxide ion ions is formed; The formation of Hydroxide ion ions causes a reaction similar to that of sodium hydroxide, and it is difficult to induce the formation of charged+2 complexes and ammonia ligands with Coordination number of 4.