Key Inclusions
A general overview of cell therapies, along with information on the key challenges and factors influencing the cell therapy manufacturing market. It provides details on the automation tools and technologies being utilized for improving manufacturing processes and the future prospects in this domain.
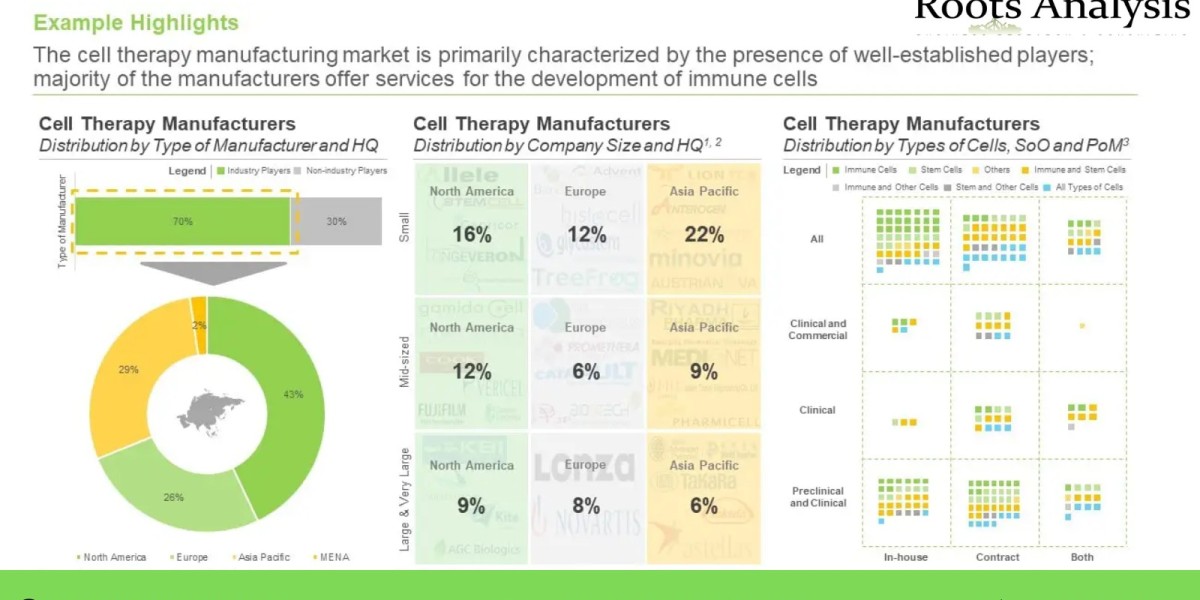
A detailed review of the overall market landscape of players engaged in the manufacturing of cell-based therapies, along with information on type of cells manufactured (including immune cells (T cells, dendritic cells, NK cells), stem cells (adult stem cells, human embryonic stem cells and induced pluripotent stem cells) and others), source of cell (autologous and allogeneic), scale of operation (preclinical, clinical and commercial), purpose of production (fulfilling in-house requirements and contract services), manufacturing capabilities / services offered (RD, cell culture development, quality testing, packaging, cell banking, supply chain management services, and regulatory services), location of headquarters and their respective manufacturing facilities.
A discussion on cell therapy manufacturing related regulations across various geographies, including North America (focusing on the US), Europe and Asia (focusing on Japan and China), featuring an analysis of the diverse certifications / accreditations awarded to the manufacturing facilities by important regulatory bodies across the globe.
An overview of the various roadmaps published by different agencies across the globe in order to provide strategies to advance cell therapy manufacturing processes.
An elaborate discussion on the role of technology automation in order to improve the current manufacturing methods, along with a comparative (qualitive) analysis of the cost differences between manual and automated processes.
Elaborate profiles of key industry players that offer contract manufacturing services for cell therapies at the clinical and / or commercial scales of operation. Each profile features a brief overview of the company, along with details related to its cell therapy manufacturing service portfolio, its manufacturing capabilities and facilities, recent partnerships and an informed future outlook.
Elaborate profiles of non-industry players that offer contract manufacturing services for cell therapies, featuring an overview of the organization, along with details related to its cell therapy manufacturing service portfolio and manufacturing facilities.
A discussion on the role of non-profit organizations, featuring a list of organizations that are actively involved in the development and production of cell-based therapies, across different geographical locations, along with information on various international / national societies that help in disseminating knowledge about the advancement of these therapies to the general community.
An analysis of completed, ongoing and planned clinical trials, based on several relevant parameters, such as trial registration year, enrolled patient population, trial status, trial phase, type of sponsor / collaborator, patient segment, target therapeutic area, study design, most active industry and non-industry players (in terms of number of clinical trials conducted) and regional distribution of trials.
A detailed analysis of the recent partnerships and collaborations inked by players focused on the manufacturing of cell-based therapies, during the period 2016-2022, based on several relevant parameters, such as the year of agreement, type of partnership model adopted, type of cells manufactured and scale of operation.
An analysis of the various expansion initiatives undertaken by service providers engaged in this domain in order to augment their respective cell therapy manufacturing capabilities, during the period 2016-2022, based on several relevant parameters, such as year of expansion, type of cell manufactured, scale of operation, purpose of expansion (facility expansion and new facility), location of expanded manufacturing facility, and most active players (in terms of number of expansion initiatives undertaken).
An in-depth analysis of the various cell therapy manufacturing focused initiatives undertaken by big pharma players, based on several relevant parameters, such as number of initiatives, year of initiative, purpose of initiative, type of initiative, scale of operation and type of cell manufactured.
An estimate of the overall, installed capacity for the manufacturing of cell-based therapies, based on information reported by various industry stakeholders in the public domain, highlighting the distribution of the available capacity on the basis of scale of operation (clinical and commercial), company size (small, mid-sized and large firms) and key geographical regions (North America, Europe and Asia Pacific).
Informed estimates of the annual commercial and clinical demand for cell therapies (in terms of number of patients), based on type of cell therapy and key geographical regions.
A detailed analysis of various factors that are likely to influence the price of cell-based therapies, featuring different models / approaches adopted by manufacturers in order to determine the price of their proprietary offerings.
A qualitative analysis, highlighting the various factors that need to be taken into consideration by cell therapy developers, while deciding whether to manufacture their respective products in-house or engage the services of a CMO.
An in-depth analysis of cell therapy manufacturers using three versatile representations, namely, a three dimensional grid analysis, presenting the distribution of companies on the basis of type of cell manufactured, scale of operation and purpose of production, a logo landscape, based on the type of cell manufactured, geographical location of manufacturer (North America, Europe and Asia Pacific), and type and size of organization (non-industry players, and small, mid-sized and large companies), and a schematic world map representation, highlighting the geographical location of cell therapy manufacturing facilities of both industry and non-industry stakeholders.
A collection of key insights derived from the study, including a grid analysis, two logo landscapes and two schematic world map representations highlighting various offerings and details of the cell therapy manufacturing service providers across different continents.
A discussion on affiliated trends, key drivers and challenges, which are likely to impact the industrys evolution, under an elaborate SWOT framework, along with a Harvey ball analysis, highlighting the relative effect of each SWOT parameter on the overall market dynamics.
Insights generated in a market-wide survey, featuring inputs solicited from experts who are directly / indirectly involved in the development and / or manufacturing of cell-based therapies.
Informed estimates of the existing market size and the future opportunity for cell therapy manufacturing market, over the next decade. Based on parameters, such as number of ongoing / planned clinical studies, cell therapy manufacturing costs, target patient population, and anticipated adoption of such products, we have provided informed estimates on the evolution of the market in the short to mid-term and mid to long term, for the period 2022-2035.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
Type of Cell Therapy
T cell therapies
Dendritic cell therapies
NK cell therapies
Stem cell therapies
Source of Cell
Autologous
Allogeneic
Scale of Operation
Clinical
Commercial
Purpose of Manufacturing
In-house Manufacturing
Contract Manufacturing
Geographical Regions
North America
Europe
Asia Pacific
Rest of the World
Key Questions Answered
What is the current, annual, global demand for cell-based therapies? How is the demand for cell-based therapies likely to evolve over the next decade?
What is the current, installed contract manufacturing capacity for cell therapies?
What are the key parameters governing the price of cell therapies?
What are the key recent developments (such as partnerships and expansions) that have been undertaken in the field of cell therapies?
What kind of partnership models are commonly adopted by stakeholders engaged in the cell therapy manufacturing domain?
What are the different types of initiatives undertaken by big pharma players for the manufacturing of cell therapies in the recent past?
What are the different types of automated technology platforms that are available to be adopted for the development and manufacturing of cell therapies?
Who are the key players (industry / non-industry) engaged in the manufacturing of cell-based therapies across the world?
What is the estimated total capital expenditure required to set up a cell therapy manufacturing facility?
What are the key factors influencing the make (manufacture in-house) versus buy (outsource) decision related to cell therapies?
How is the current and future market opportunity for cell-based therapies likely to be distributed across various key market segments?
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html
You may also be interested in the following titles:
About Roots Analysis
Roots Analysisis a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415