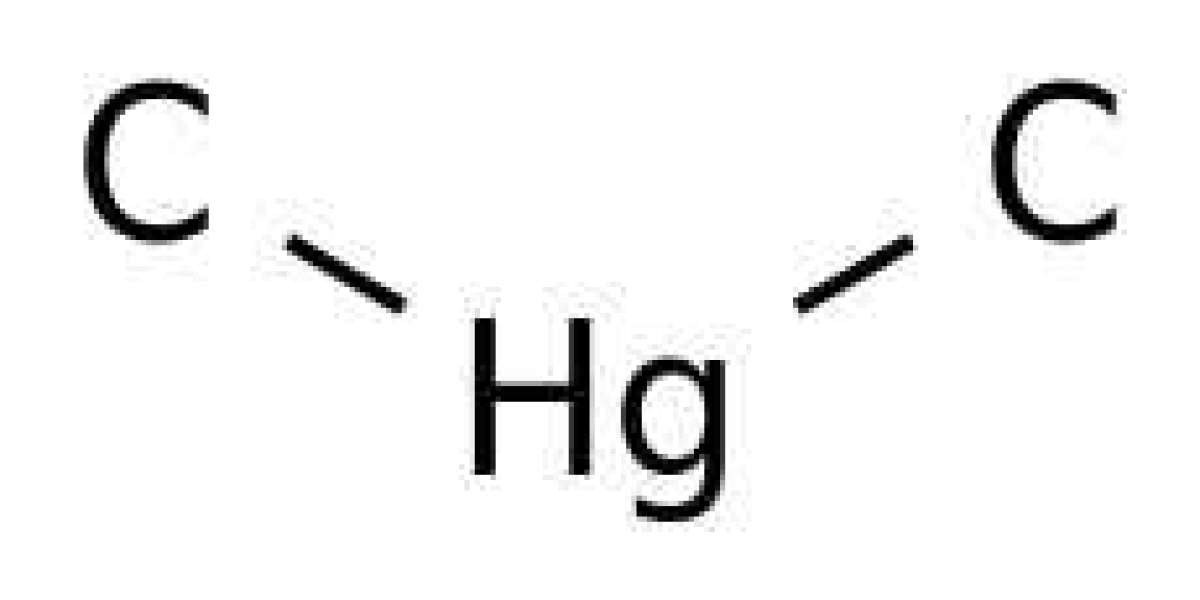
Underlying formation pathways of dimethylmercury ((CH3)2Hg) in the ocean are unknown. Early work proposed reactions of inorganic Hg (HgII) with methyl cobalamin or of dissolved monomethylmercury (CH3Hg) with hydrogen sulfide as possible bacterial mediated or abiotic pathways. A significant fraction (up to 90%) of CH3Hg in natural waters is however adsorbed to reduced sulfur groups on mineral or organic surfaces. We show that binding of CH3Hg to such reactive sites facilitates the formation of (CH3)2Hg by degradation of the adsorbed CH3Hg. We demonstrate that the reaction can be mediated by different sulfide minerals, as well as by dithiols suggesting that e.g. reduced sulfur groups on mineral particles or on protein surfaces could mediate the reaction. The observed fraction of CH3Hg methylated on sulfide mineral surfaces exceeded previously observed methylation rates of CH3Hg to (CH3)2Hg in seawaters and we suggest the pathway demonstrated here could account for much of the (CH3)2Hg found in the ocean.
Dimethylmercury is a volatile and highly toxic form of mercury (Hg). It appears to be ubiquitous in marine waters and has been found in deep hypoxic oceanic water, coastal sediments and upwelling waters and in the mixed layer of the Arctic ocean. Reported concentrations of (CH3)2Hg in marine waters range from 0.01–0.4 pM and (CH3)2Hg has been found to constitute a significant fraction (up to 80%) of the methylated Hg pool (CH3Hg + (CH3)2Hg). The role of (CH3)2Hg in the biogeochemical cycle of mercury and its bioaccumulative potential, is not well known. However, for oceanic systems and for the marine boundary layer, it has been suggested that degradation of (CH3)2Hg is an important source of CH3Hg.
Diane Stearns, one of Wetterhahn's post docs, visited her after she became comatose. Her words were chilling: "She was thrashing about. Her husband saw tears rolling down her face. I asked if she was in pain. The doctors said it didn't appear that her brain could even register pain." I have written before about chemicals that are to be avoided if possible, such as t-butyllithium and fluorine. These are a teaspoon of honey compared to dimethylmercury. However, not all mercury-containing chemicals behave like dimethylmercury. Far from it. To understand this accident, which many consider one of the worst laboratory incidents ever, we need to talk about different forms of mercury. They range from almost harmless to what is arguably the most dangerous chemical of all. All mercury is not created equal.