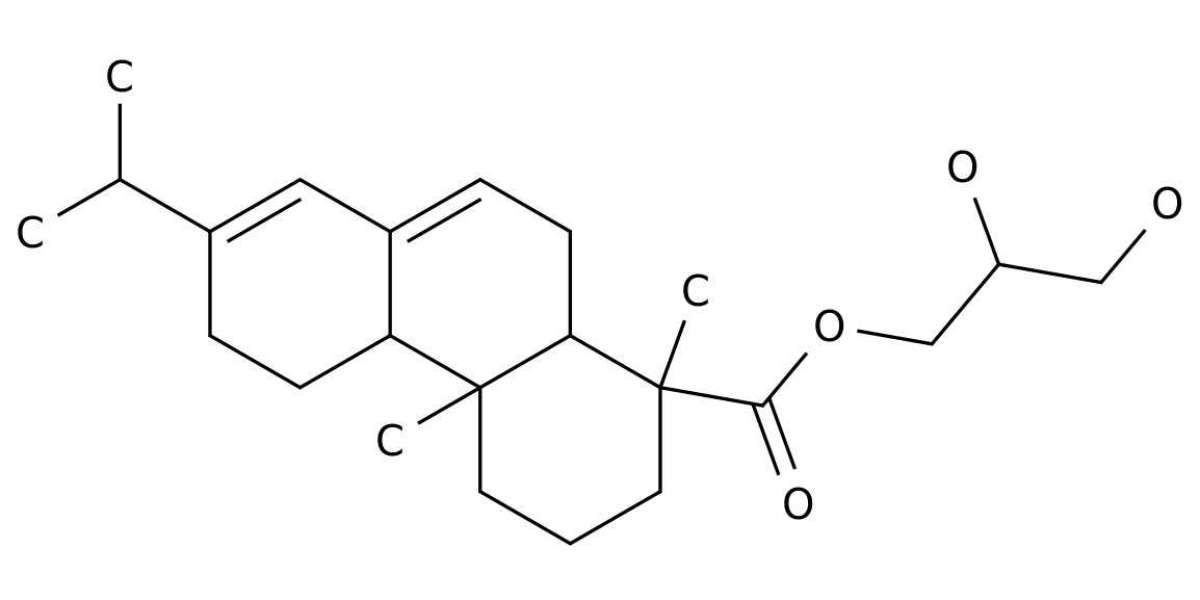
The FDA approved GEWR for use in beverages in the early 1960s. During the review and approval process, a suggestion was submitted that the product be referred to by the broader term of “glycerol ester of rosin.” Since available data didn’t support this proposal, this suggestion was rejected, and “glycerol ester of wood rosin” was adopted. This was an early acknowledgement that the source of the feed rosin was important and that all glycerol esters of rosin could not be considered equivalent based on superficial similarities. The FDA ultimately approved GEGR use in beverages in 2005, followed by Health Canada in 2010, but multinational regulatory agencies have not followed suit due to incomplete compositional and toxicological information.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) carried out a comprehensive evaluation of GEWR produced from P. palustris and P. elliottii beginning in 1974. JECFA took a conservative approach to evaluating and approving this material as a food additive, and the original manufacturer of GEWR initiated a long-term program of analytical and toxicological testing at JECFA’s request. From the beginning, JECFA emphasized the need to more fully characterize the material from which the glycerol ester of rosin was prepared, including specifying the species of pine tree used. The rigorous evaluation process culminated in 1991 with specifications for GEWR under International Numbering System (INS) 445.
In 2008, FDA requested that JECFA consider the extension of INS 445 approvals to GEGR. The basis was a claim of chemical equivalence between GEGR and GEWR to justify relying on the extensive toxicological testing done on the original GEWR product. JECFA maintained a consistent, science-based approach in its evaluation of this request. During evaluation of the GEGR petition, the committee noted that the chemical composition of GEGR varies depending on the pine species, geographical differences, and the techniques used in the processes of rosin production. JECFA noted that limited data was available on the variability of the resin acid composition of GEGR in commerce and that complete information on the composition and ester distribution of GEGR had not been submitted. Therefore, officials were not able to claim similarities to GEWR. JECFA was unable to establish specifications and an allowable daily intake for GEGR due to the lack of information.
GEWR is the only glycerol ester of rosin approved for use in beverages by the European Food Safety Authority (EFSA). EFSA was petitioned by TR Chemicals, Inc., in 2008 for the approval of GEGR as a beverage-weighting agent under E 445. A claim of chemical equivalence was put forth by the petitioner to justify use of the original GEWR toxicity test data. The EFSA Scientific Panel on Food Additives and Nutrient Sources Added to Food (ANS) reviewed the proposal and concluded that the available data was too limited to evaluate GEGR’s safety as a food additive. The panel couldn’t verify chemical equivalence of GEGR and GEWR due to the lack of critical information on composition, so toxicological data for GEWR could not be used for read-across to GEGR.