Researchers at Dartmouth College are clarifying the early chemical reactions in organic sediments that ultimately became the main source of natural gas and oil, the Marcellus shale.
The research results were published in the Journal of Geochemistry and Cosmochemistry. This study extends an earlier study by the Dartmouth College team.
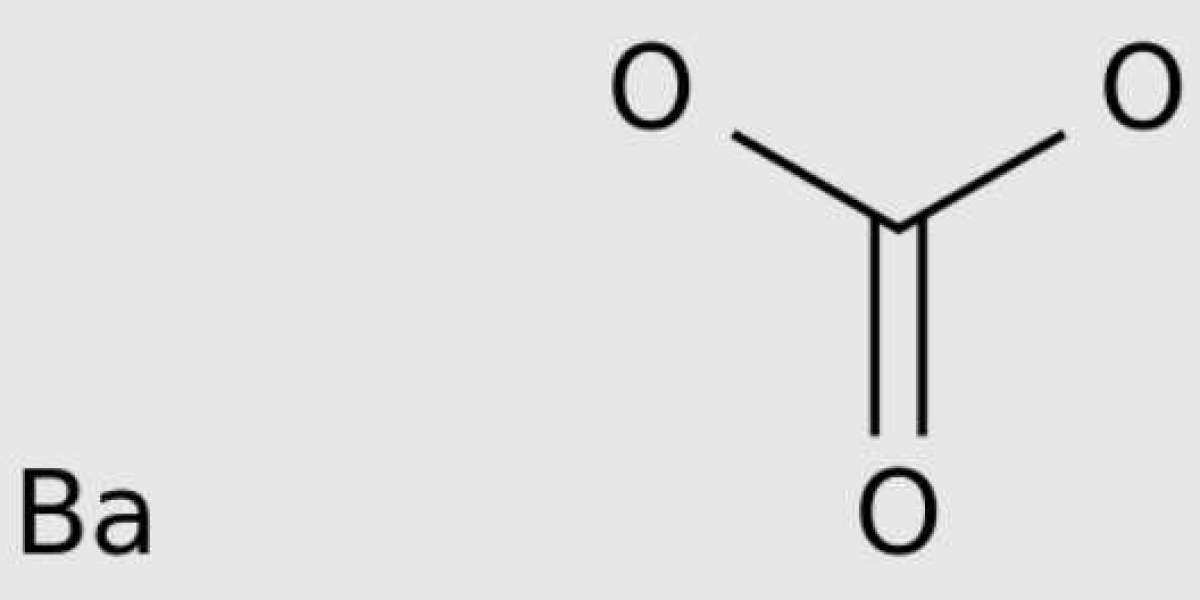
During hydraulic fracturing, the water injected into the shale formation returns with extremely high total dissolved solids and high concentrations of toxic metal barium. It is speculated that part of the reason for these hazardous waste waters is that chemicals are introduced into the fresh water when the injected fresh water is mixed with naturally occurring high salt brine in the rocks. In their early research, however, researchers found that a chemical reaction between injected fresh water and the fractured shale itself resulted in the leaching of barium directly from the fractured rock.
In this new study, researchers solved the fundamental questions of when, why, and how the Marcellus shale initially enriched barium. They studied the chemistry, mineralogy, and sulfur isotope composition of this two inch thick core in detail. This is a special sample because each gram of rock contains about 5000 micrograms of barium. These samples are concentrated only in a few depth intervals of the Marcellus shale. This sample provides researchers with information on how, when, and where barium containing minerals (barite) transformed into iron sulfide (pyrite) during the Devonian period when organic rich marine sediments were converted into shale.
Researchers have found that barite particles are "bitten" by pyrite because the latter replaces the former through a series of complex reactions that can only occur at very specific depth intervals where sulfate (barite) and sulfide (pyrite) coexist. This interval, known as the sulfate methane transition zone, has been found in relatively new sediments, but not in sediments 400 million years ago.
"Our new research provides insights into the general nature of barium mobilization, as barite dissolves in organically rich sediments and redistributes in clay minerals," said Mukul Sharma, a professor of earth sciences and senior author.