Fortification of food with iron is considered to be an effective approach to counter the global health problem caused by iron deficiency. However, reactivity of iron with the catechol moiety of food phenolics leads to discolouration and impairs bioavailability. In this study, we investigated the interplay between intrinsic and extrinsic factors on food discolouration caused by iron-catechol complexation. To this end, a three-level fractional factorial design was implemented. Absorbance spectra were analysed using statistical methods, including PCA, HCA, and ANOVA. Furthermore, a direct link between absorbance spectra and stoichiometry of the iron-catechol complexes was confirmed by ESI-Q-TOF-MS. All statistical methods confirm that the main effects affecting discolouration were type of iron salt, pH, and temperature. Additionally, several two-way interactions, such as type of iron salt × pH, pH × temperature, and type of iron salt × concentration significantly affected iron-catechol complexation. Our findings provide insight into iron-phenolic complexation-mediated discolouration, and facilitate the design of iron-fortified foods.
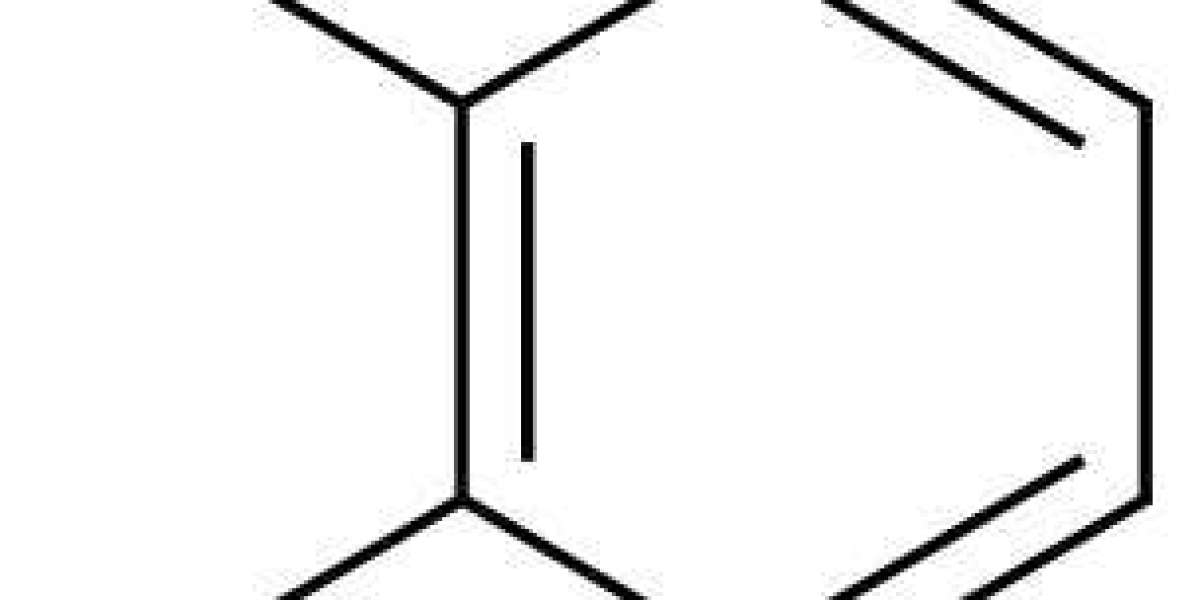
To date, chemical interactions between phenolics and iron, combining redox processes and complex formation, have not been fully elucidated. Oxidation of the 1,2-dihydroxybenzene moiety of phenolics in presence of ferric iron (Fe3+) leads to the formation of quinones that polymerise to form brown-coloured compounds. However, Fe3+-catalysed oxidation reactions of phenolic derivatives and catechol are very slow. On the other hand, complexation reactions by the formation of a coordinate bond between Fe3+ and deprotonated catechol, lead to fast and intense discolouration. Discolouration upon iron-catechol complexation results from the intense ligand-to-metal charge transfer (LMCT) absorbance band, typically observed between 380–800 nm. Deprotonation of the catechol moiety is required for iron binding, thus stoichiometry and colour of the iron-catechol complexes is pH dependent, as the complexing capacity of catechol increases at higher pH. The pKa for the first hydroxyl group of catechol is 9 and of the second hydroxyl group 13.013. However, if iron is present, the deprotonated state is stabilised and thermodynamically more favourable, leading to an apparent pKa of 5–8.
Iron-mediated oxidation of catechol is a slow process9, therefore, the main mechanism responsible for discolouration of iron-fortified, phenolic-rich food products is expected to be iron-catechol complex formation. This study, therefore, focusses on discolouration resulting from fast complexation between iron and catechol. Understanding the interplay between intrinsic and extrinsic factors on iron-catechol complexation is required to design iron-fortified products while preventing discolouration and ensuring bioavailability of iron. The extrinsic factors of interest for food systems are temperature and humidity. Intrinsic factors expected to influence the iron-catechol complexation are: pH, the type of iron salt, iron concentration, ratio iron:catechol, ionic strength, and presence of taste enhancers. The effect of several factors on iron-catechol complexation is currently unknown or unclear due to contradictory reports in literature. Additionally, two-way interactions between the aforementioned factors have not yet been investigated.