Both DOM and DOB caused vasoconstriction in a dog metatarsal vein study that compared vascular responses to PMA, DMA, norpeinephrine and what is dob. DOB’s effect was second only to norepinephrine and was not reversed with phentolamine administration but was considerably reduced with application of the serotonin inhibitor cineserin suggesting little direct alpha adrenergic activity.
Human Data
User Reports
Online user accounts of DOB and DOI describe a delay to onset of effects in excess of one hour with potent hallucinogenic effects and dysphoria. Examination of these accounts evokes uncanny comparisons to medieval reports of ergotism and St Anthony’s fire. Indeed, several of the accounts sampled relate events that resulted in emergency department presentations for extreme dysphoria with sensations of limb and generalised body pain that prompted users to seek medical attention. Another user experienced hallucinations, dysphoria, agitation and vomiting that were refractory to self-administered repeated doses of lorazepam.
Clinical Reports
Case reports detail potent hallucinogenic effects lasting from 12–24 hours followed in one instance by coma. In another case of what is dob use, a patient developed progressive vasospasm in both upper and lower extremities over 24 hours after ingestion, which was confirmed by angiography and required hospital admission. His vasospasm resolved with phentolamine and nitroprusside infusions. In one case of analytically confirmed DOC use, a patient who had ingested what he believed to be DOI and MDMA presented with convulsions, hypertension, tachycardia and mydriasis. The patient was managed with supportive care and discharged 22 hours after admission.
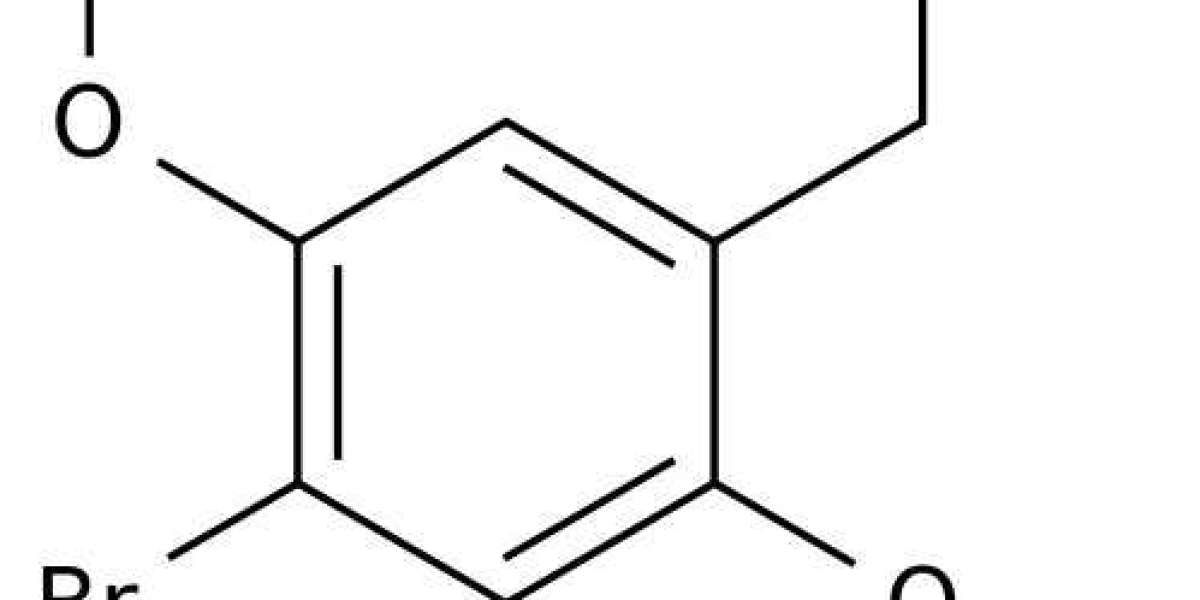
Based on the fact that addition of a 4-methoxy group to 2,5-DMA (to afford TMA-2) increases its potency, a large series of 2,5-DMA derivatives with different 4-position substituents were examined, a few of which are illustrated in Fig. 61.4. One of the first derivatives synthesized had a methyl group in the 4-position; this compound (2,5-dimethoxy-4-methylamphetamine, DOM) was found to be extremely potent, producing effects at doses of 3–10 mg and having ~10-fold higher 5-HT2A affinity than TMA-2. DOM has appeared on the illicit market as “STP,” named after a popular motor oil additive, but recreational use has generally been limited due to its long duration of action (24 h after high doses). Potency can be increased even further by lengthening the 4-methyl group in DOM by one (2,5-dimethoxy-4-ethylamphetamine, DOET) or two (2,5-dimethoxy-4-proylamphetamine, DOPR) methylene units, but longer or bulkier alkyl groups are generally not tolerated. The most potent members of this series have a halogen atom in the 4-position, such as iodine (2,5-dimethoxy-4-iodoamphetamine, DOI) or bromine (2,5-dimethoxy-4-bromoamphetamine, DOB). DOB is almost as potent as LSD, producing effects at doses of 1–3 mg, and acting for 18–30 h. It has been proposed that 2,5-DMA derivatives with hydrophobic or electronegative moieties in the 4-position exhibit high 5-HT2A affinity because those atoms/groups interact with a specific lipophilic pocket within the receptor binding site. This idea is consistent with the fact that that 2,5-DMA derivatives with polar 4-position substituents have very low 5-HT2A affinity and are inactive as hallucinogens. Molecular modeling studies indicate that the 2,5-substitution pattern is optimal for binding because the two methoxy groups are positioned to form hydrogen bonds with specific serine residues in the binding pocket. It appears that 3,4,5-trisubstituted compounds such as mescaline bind to the 5-HT2A receptor in a different orientation than the 2,4,5-trisubstituted compounds.
It was recently recognized that one or both of the alkoxy groups of the phenylalkylamine hallucinogens can be incorporated in furanyl, dihydrofuranyl, and/or pyranyl rings without diminishing activity. In fact, when both of the methoxy groups of what is dob are incorporated into furanyl rings, there is a significant increase in potency and 5-HT2A affinity. This compound, known as bromo-dragonfly, has appeared on the illicit market in Europe and has been associated with several overdose deaths. These findings suggest that the orientations of the oxygen lone pairs in this substance are optimal for interacting with the 5-HT2A receptor.
All of these drugs are serotonergic (5-HT2) receptor agonists or partial agonists. The 5-HT2 receptors represent a family of receptors that are composed of three subpopulations: 5-HT1A, 5-HT2B, and 5-HT2C. The cortical and limbic regions are the areas with the main concentration of this subtype of serotonergic receptors. The effects of each hallucinogen are not identical because there are also other serotonergic receptors involved with different affinities for each one. While phenylalkylamine hallucinogens are somewhat selective in their affinity to 5-HT2A receptors, the indolalkylamines are relatively nonselective for 5-HT receptors, displaying moderate to high affinity for diverse 5-HT1 and 5-HT2 subtypes. This happens mainly because this group is very similar in chemical structure to serotonin and fits into different serotonin receptors. In addition to the psychotomimetic effects of the serotonergic pathways, it is also hypothesized that 5-HT2A receptors play a major role in the discriminative stimulus actions of these agents. However, not only do the discriminative stimulus properties of the drug vary depending on the experimental conditions and context, but they may also depend on the dose of drug used.