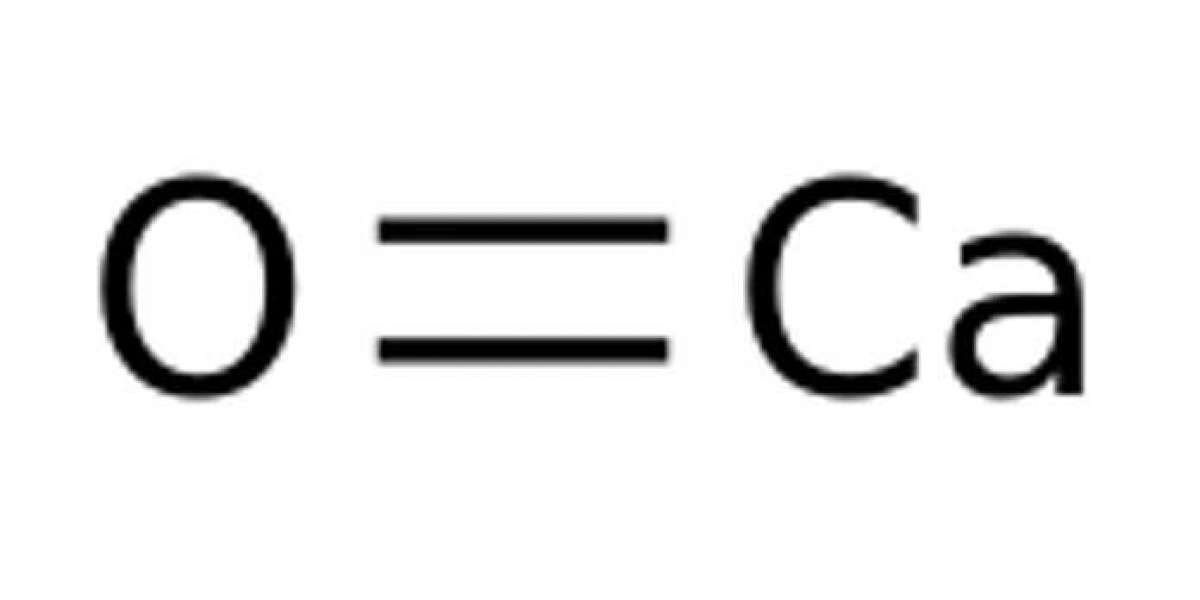CaO has been known since ancient times. The Roman writer Cato the Elder (234 - 149 BC) mentioned one method of making the compound in 184 BC. By the early fifteenth century, many Europeans were using CaO (generally referred to as lime) in the construction of buildings. The Scottish chemist Joseph Black (1728–1799) performed some of the earliest scientific studies of what is CaO. He found that when the compound is exposed to air, it combines with carbon dioxide to produce calcium carbonate.
Calcium oxide is a chemical compound that has colourless, odourless properties and was used since the early times. The formula for calcium oxide is CaO. It is an amorphous substance that is in a crystalline or powdery solid form. CaO is also called quick lime, caustic lime or burnt lime. In its pure form, CaO is white or off grey in colour. On the other hand, it is yellow or brownish in colour in the presence of impurities, such as iron, magnesia, silica or alumina. CaO also exists in the colours reds and muted browns.
The main primary elements which constitute calcium oxide are calcium and oxygen.It is prepared by heating calcium carbonate in a distinct lime kiln to about 500°C to 600°C, decomposing it into the oxide and carbon dioxide.This process of obtaining burnt lime is called calcification. It starts with decomposing the natural components at high temperatures while maintaining they do not reach the melting point. This process is done by heating them at temperatures ranging from 1070 degrees Celsius to 1270 degrees celsius. Calcium oxide is used in industries that make porcelain and glass. It is also used for purifying sugar, in preparing bleaching powder, calcium carbide, and calcium cyanamide. Its other uses are in water softeners, mortars, and cement. Calcium oxide (CaO), is generally known as quicklime or burnt lime, it is a commonly used chemical compound. It is solid at room temperature. The broadly used word lime means calcium-containing inorganic materials, in which oxides and hydroxides of calcium, magnesium, aluminium, silicon, and iron are present. By contrast, quicklime precisely applies to the single chemical compound CaO. But commercial lime frequently contains impurities. CaO is also widely used in medicines and pesticides. Due to alkali being available in affordable amounts, CaO is one of the essential ingredients in making caustic soda. It is also used in the manufacturing of steel, paper, and cement.
The second most vital use of what is CaO is in pollution control devices. Smoke that ejects from any industry’s smokestack contains high amounts of sulfur and nitrogen. When sulfur and nitrogen are combined with water, it morphs into new substances, such as nitric acid and sulphuric acid. To prevent such harmful chemical compositions from getting in contact with nature, machines called scrubbers are installed. These devices have large compounds of CaO which help in neutralising the high amounts of sulphuric acid and nitric acid.
Benefits Of Calcium In The Human Body
Calcium is mainly needed for building strong bones and maintaining good oral health. 99% of calcium is found in bones and teeth. It is essential for the growth, development, and maintenance of the body. Calcium also helps in maintaining the activity of the blood and ensuring its smooth flow in the whole body. The presence of adequate calcium in the body results in lower blood pressure, high amounts of energy and promotes effective rejuvenation of the skin and bones. It also helps in muscle contraction and improves cholesterol functioning.
Interesting Facts about CaO
CaO is frequently used to "lime" lake waters that have been acidified by acid rain; it reacts with them and neutralizes acids in the lake. It is also formed when nitric and sulphuric acid in acid rain is carried to earth by rain, sleet, snow, and other methods of precipitation. When CaO is heated near its melting point, it gives off a bright white light. In the years before electricity was discovered for lighting, particularly during the second half of the 19th century it was used as a limelight source, heated lime was used to create the bright lights required to illuminate stage productions. Because it was thought to increase the speed in the decomposition of soft tissue, quicklime has historically been used in the funeral of diseased animals and humans. For instance, the bodies of plague victims in London in 1666 were instructed to be buried in quicklime.
Health Risk
Contact with what is CaO can cause injury to the skin, nose, eyes, and respiratory system. People who use the product in their line of work or at home for (garden purposes) for example, people who are working must be extremely cautious not to breathe in, swallow, or otherwise come into contact with the chemical. If such contact occurs, it should be washed off completely with water and asked for medical assistance.