Although there are several treatment options available to control disease progression and keep malignant cells from spreading throughout the body, lasting remission is difficult to achieve. In this context, immunotherapies, a relatively recent addition to the gamut of anticancer interventions, have demonstrated significant promise. Amidst the current initiatives undertaken to develop more targeted anti-cancer therapies, CAR-T cell therapies have emerged as a promising option, owing to their ability to eradicate tumor cells from the body with minimal treatment-related side effects.
CAR-T Cell Therapies – Current Market Landscape
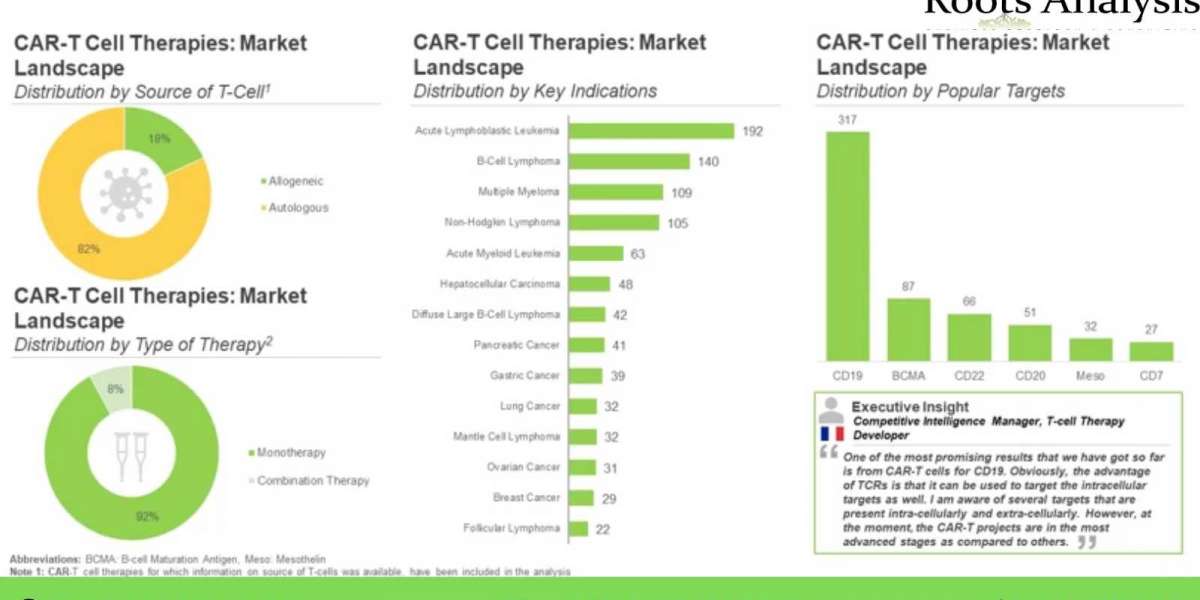
Presently, over 170 companies are engaged in the development of more than 970 early and late-stage T-cell therapies, worldwide. More than 80% of the therapy candidates, which are being developed to target a range of disease indications, are autologous in nature. Further, CD19 and BCMA have emerged as the most popular target antigens.
CAR-T Cell Therapies – Partnerships and Collaborations
A rise in partnerships, in the recent past, involving both international and indigenous stakeholders, validate the growing interest in this domain; maximum number of such deals were signed in 2021. Majority of the partnership instances are intracontinental. Additionally, close to 50% of the total number of partnerships have been signed between the players based in North America. Further, for majority of the intercontinental agreements, companies headquartered in Europe have collaborated with players based in North America.
CAR-T Cell Therapies – Funding and Investments
Several investors, having realized the opportunity within this upcoming segment of cancer immunotherapy, have invested close to USD 25 billion, across 260+ instances, during the period 2000-2022
CAR-T Cell Therapies – Patent Analysis
Majority of the patents in this domain are patent applications (84%), followed by granted patents (13%). In addition, most of the patents (35%) were filed / granted in Asia-Pacific. This is followed by patents filed / granted in North America (30%).
CAR-T Cell Therapies – Market Forecast and Opportunity Analysis
In the long-term, the overall projected opportunity is likely to be well distributed across key market segments, including type of therapy, indications, target antigen, key players and geographical regions; the market is expected to grow at a CAGR of ~20% by 2035.
For additional details, please visit
https://www.rootsanalysis.com/blog/car-t-cell-therapies/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
Biologics Fill Finish Services Market (3rd Edition), 2022-2035
Bioavailability Enhancement Technologies and Services Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415