Background
Acetylacetone is a commercially bulk chemical with diverse applications. However, the traditional manufacturing methods suffer from many drawbacks such as multiple steps, harsh conditions, low yield, and environmental problems, which hamper further applications of petrochemical-based acetylacetone. Compared to conventional chemical methods, biosynthetic methods possess advantages such as being eco-friendly, and having mild conditions, high selectivity and low potential costs. It is urgent to develop biosynthetic route for acetylacetone to avoid the present problems.
Results
The biosynthetic pathway of acetylacetone was constructed by reversing its biodegradation route, and the acetylacetone was successfully produced by engineered Escherichia coli by overexpression of acetylacetone-cleaving enzyme from Acinetobacter johnsonii. Several promising amino acid residues were selected for enzyme improvement based on sequence alignment and structure analysis, and the acetylacetone production was improved by site-directed mutagenesis of Dke1. The double-mutant strain presented the highest acetylacetone-producing capacity which is 3.6-fold higher than that of the wild-type protein. Finally, the strain accumulated 556.3 ± 15.2 mg/L acetylacetone in fed-batch fermentation under anaerobic conditions.
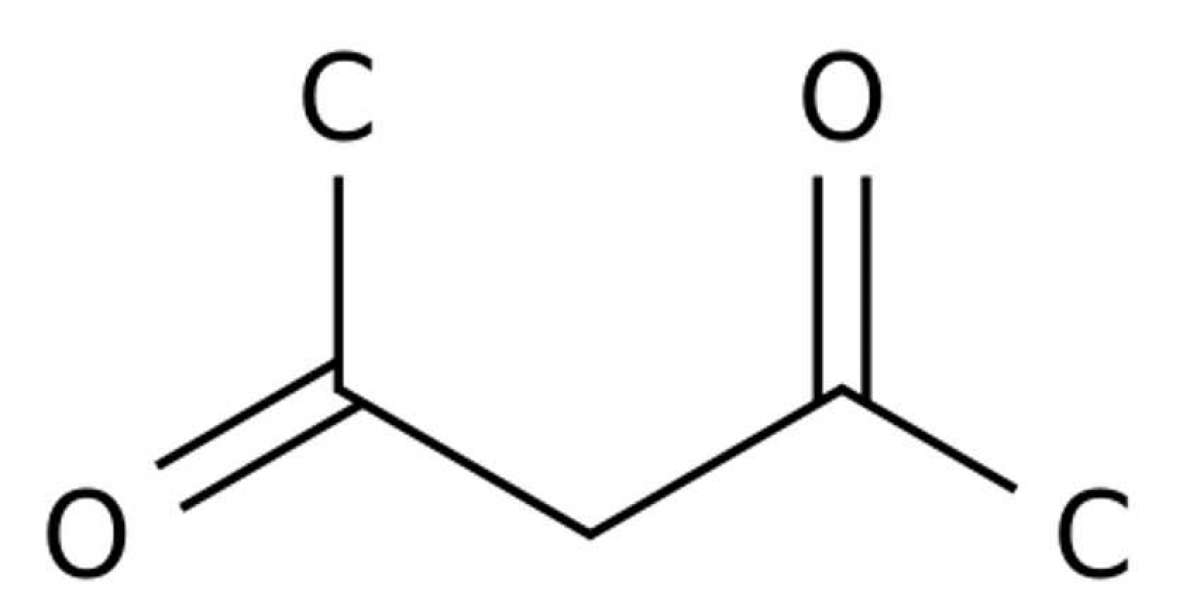
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Traditionally, acetylacetone is manufactured through chemical routes using acetone and ketene, which is produced by pyrolysis of acetone or acetic acid at a temperature of 700–800 °C, with carbon monoxide, methane, hydrogen formed as by-products. In specific, esterification of ketene and acetone forms isopropenyl acetate, in the presence of a strong acid catalyst. Then, IPA is transformed into acetylacetone at 500–600 °C with metallic molybdenum as a catalyst, whereby the yield is only about 45%. In conclusion, the chemical routes suffer from drawbacks such as multiple steps, harsh conditions, low yield, and environmental problems, which hamper further applications of petrochemical-based acetylacetone. To address the issue, new methods need to be developed for acetylacetone preparation. Compared to conventional chemical methods, biosynthetic methods are expected to have advantages such as being eco-friendly and having mild conditions, high selectivity and low potential costs, and have been used to produce numerous products, e.g., bio-based chemicals, pharmaceuticals, biopolymers. For acetylacetone, the theoretical yield is as high as 1.5 mol/mol glucose by bioconversion. Predictably, low cost will also be obtained thanks to the cheap carbon source and the high yield.
Limited studies indicate that acetylacetone is biodegradable. A strain of Acinetobacter johnsonii was isolated and proved to have the ability to utilize acetylacetone as a sole carbon source. To reveal the decomposition mechanism, a novel C–C bond-cleaving enzyme, acetylacetone-cleaving enzyme, was found and purified from A. johnsonii. The Dke1 enzyme can activate oxygen to cleave acetylacetone into acetate and methylglyoxal, followed by the conversion of methylglyoxal into lactate by glyoxalase. However, the natural biosynthesis of acetylacetone has not yet been reported. In previous studies, some non-natural products had been bio-synthesized by reversing their biodegradation pathway. An artificial biosynthetic pathway to methylacetoin was constructed by redirecting the methylacetoin biodegradation, and the titer achieved at 3.4 g/L by enzyme screening and metabolic engineering. The direct biocatalytic route of 1,4-butanediol mirroring its biodegradation pathway was reported with a production of 18 g/L. In addition, the engineered reversal of the β-oxidation cycle was adopted for fatty-acid-derived compounds’ biosynthesis, and a series of short-, medium-, and long-chain products were obtained at high yields. These successful cases provided a possible strategy to our research for acetylacetone biosynthesis.
In this work, we established for the first time the biosynthetic pathway of acetylacetone from fermentable sugars inspired by the known acetylacetone biodegradation pathway. It was proved that the acetylacetone decomposition process catalyzed by Dke1 was reversible. The Dke1 activity was improved by rational design, resulting in enhanced acetylacetone production under shake-flask conditions, and the underlying mechanism was proposed. Fed-batch fermentation was conducted to evaluate the potential for large-scale production.