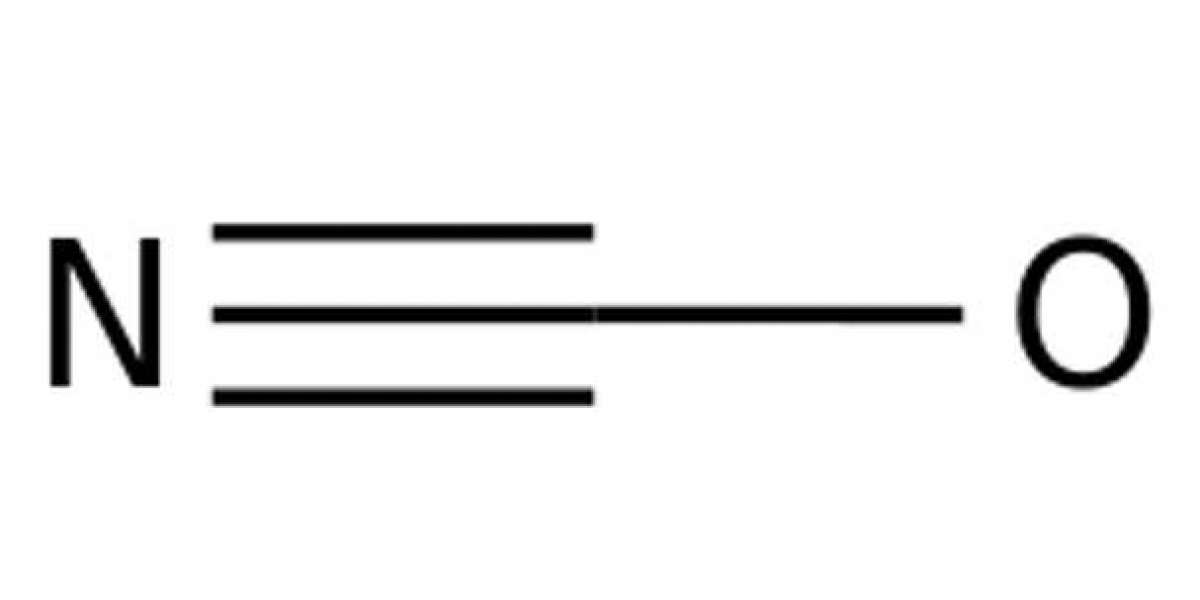Various reactions of cyanic acid and the cyanate ion have been examined. Cyanic acid, in the presence of added hydrochloric or nitric acid, decomposes quantitatively according to the equation.The rate constant for this reaction was measured over a range of temperature and ionic strength, and was found to be 0.86 mole at unit ionic strength. The activation energy is 144 kcal. The effect of ionic strength on the reaction with hydrochloric acid closely parallels that on the activity coefficients of the acid itself. Without added acid cyanic acid decomposes by a first order reaction, followed by a rapid second stage. This reaction has a rate constant of, and an activation energy.
Past worlters have established with reasonable certainty the qualitative aspects of the decomposition reactions of cyanic acid and the cyanate ion. However, quantitative measurements have not been too systematic, and it is hoped that the present work will give a more complete picture. Besides the decomposition reactions in aqueous solution, which are really hydrolyses, some observations are included on the oxidation of the cyanate ion.
Cyanuric acid only results to any great extent in concentrated solution. The ions could react with cyanate ions to give urea; but this reaction is much slower, so that in acid solution it is negligible. Quantitative measurements on this reaction are rather limited. Report a reaction first order with respect to cyanic acid, and with a rate constant. We have studied this reaction over a range of conditions with the following results.
The ammonia formed was estimated by distilling it into a ltnown amount of standard acid, and titration. The distillatioil was brief enough so that only a very small amount of ammonia was formed by decompositioil of cyanate during this operation; howvever as this reaction was also measured, this error could be allowed for. The solution was always distilled for the same length of time. Traces of ammonium ion initially present in the cyanate (or elsewhere) were estimated in a reagent blank run. The cyanate initially present was calculated from the strengths of the stock solutions, which were determined by silver nitrate titration. At subsequent times it was assumed that the cyanate concentration was given by the initial concentration minus the ammonium ion formed. It was checlted that if the reaction was allowed to go to virtual completion the ammonium ion formed was equal to the initial cyanate.
This is a method of estimating cyanate in the absence of interfering substances, and the results agreed with those of silver nitrate titration. Hence side reactions during the titration with acid are negligible. This reaction was examined at various temperatures and ionic strengths. Results all at an ionic strength of 1.0. This ionic strength is derived on the assumption that no cyanic acid is ionized; the value for the ionization constant obtained later shows that the fraction ionized would be less than 0.001. The reaction itself will not change the ionic strength. The next question is whether this is the same reaction as that examined. Cyanic acid itself could provide hydrogen ions; from the rate constants given above and that obtained by these authors, the observed rates would be the same if the pH were 3.5, which is a reasonable value for partially hydrolyzed cyanic acid. However if cyanic acid provided the hydrogen ions the rate would not appear to be first order.
Cyanic acid solution was prepared in the usual way by passing the gases formed by heating cyailuric acid through a red-hot tube and thence into ice-cold water. A slow stream of nitrogen was used to carry over the gas. The resulting solution of cyanic acid was used as soon as possible. It was contained in a flask immersed in a water bath of the desired temperature, and samples for analysis were talcen at intervals.